
Polymer-based, 3D-printed pericardial tissue project could save lives
Canadian Plastics
3D Printing Materials Research & DevelopmentA new three-year research project is developing biomimetic polymers that can imitate the mechanical properties of pericardial tissue, with the goal of making artificially produced implants for heart disease patients who are waiting for a donor organ.
It can be a real problem when demand outraces supply. And the problem can turn tragic when human lives are on the line.
For example, an estimated 23 million people worldwide suffer from heart disease, and that number is growing. But the number of heart transplants isn’t growing – instead, it’s holding steady at a mere 3,000 per year worldwide.
Which is why a new three-year, polymer-based research project that could help many patients who are waiting for a donor organ has the potential to be such a big breakthrough. Initiated in April 2019, the publicly funded PolyKARD project includes the participation of AdjuCor GmbH, the Fraunhofer Institute for Applied Polymer Research IAP, the NMI Natural and Medical Sciences Institute, Young Optics Europe GmbH, and pro3dure medical GmbH. Each partner brings specific expertise to the project, which is managed by German engineering association, VDI Verein Deutscher Ingenieure. If successful, the development of these polymers and the associated manufacturing technologies could stem the tide of heart disease-related deaths, which are one of the most frequent causes of death.
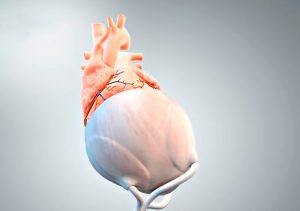
The first application of the pericardium replacement material is to produce a new type of surface for an extravascular cardiac support system using 3D printing. Image Credit: AdjuCor GmbH.
In the PolyKARD project, polymers are being developed that mimic the biological and mechanical material properties of the pericardium, the double-walled sac that contains the heart. The polymers are a less-expensive, less inconsistent alternative to the pig and cow pericardia that are currently used to replace human heart valves or to reconstruct blood vessels. The practice of using animal tissue also raises ethical and religious issues. The challenge in using polymers, however, is to develop a synthetic elastic tissue that retains its mechanical properties for many years, is 100 per cent biocompatible, and is not rejected by the immune system.
“In this project, we are developing biomimetic pericardial replacement materials that can be used, for example, for artificial pericardium, heart valves, blood vessels, stents, tendons or septum occlusions,” said Dr. Wolfdietrich Meyer, who heads the project at Fraunhofer IAP. “The special feature of these implants is that they are made of photopolymers and can be produced individually using a 3D printer or by electro-spinning. The monomers are developed as inks or resins. They only polymerize when they are irradiated with UV light.” The research team at Fraunhofer IAP is synthesizing a photo-crosslinkable material consisting of different polyurethane segments and collagen components.
The newly synthesized polymers are then tested for in vitro cytotoxicity at the NMI Natural and Medical Sciences Institute, where they are processed by means of different 3D printing methods and electro-spinning. The spinning process is used to create porous structures that can grow together with the patient’s own body tissue. The carrier substrates produced are characterized in terms of their mechanical and biological properties. Special emphasis is placed on the reproduction of the mechanical properties of the pericardium and the growth behaviour of cells.
The first application of the biomimetic polymer will be the printing of a novel surface for an extravascular cardiac support system. The system of the Munich-based AdjuCor GmbH is based on a patient-specific mechanical implant, which is positioned completely outside the blood flow (extravascular) in the pericardial cavity around the epicardial surface of both heart chambers. “A biomimetic pericardial replacement material would cause only minor immune reactions and would thus lead to a gentle healing phase – this could further shorten intensive care and hospital stays,” said heart surgeon and CEO of AdjuCor Prof. Stephen Wildhirt.
In order to be approved for clinical applications on the market in the future, both the new photopolymers and the processing methods must meet extensive requirements. For the large-scale production of photopolymers, the GMP (Good Manufacturing Practice) guidelines that ensure the quality of the production processes and environment have to be observed. Pro3dure medical GmbH has the job of upscaling the process of the photopolymers as well as the resin synthesis under consideration so that they comply with these GMP.
Young Optics Europe GmbH, meanwhile – which has processed biocompatible photopolymers for Class I and IIa medical products using its 3D printers – is developing a 3D printing system suited for the production of Class III medical products as part of the PolyKARD project.
The project is still in its early stages, but the research is already achieving promising results. “We have already successfully synthesized and printed the first elastic photourethane resins from non-toxic starting materials,” Dr. Meyer said. “In the future, we would like to apply the medical concept of wholeness even more strongly to our chemistry. We want to develop more materials based on renewable raw materials for 3D printing and electro-spinning that are biocompatible and can be processed with the highest precision”.
The first clinical studies could happen as soon as 2022.
Source: AdjuCor GmbH
